You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry help
- Thread starter whoa-dere
- Start date
Darth.Maul
Active member
Products-reactants
You have 2 CO2's whoch is 4 CO double bonds and a H2 single bond on the product side.
You have 2 CO's which gives 2 CO triple bonds and H2O2 which gives 2 OH bonds and a OO single bond.
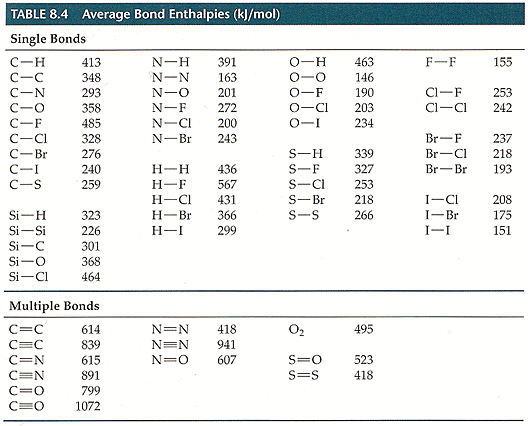
I cant read the numbers on the chart of bond enthalpies but that should be the way to figure out which numbers to use from the chart. You can draw lewis structures to determine the number of bonds between the atoms in each compound.
You have 2 CO2's whoch is 4 CO double bonds and a H2 single bond on the product side.
You have 2 CO's which gives 2 CO triple bonds and H2O2 which gives 2 OH bonds and a OO single bond.
I cant read the numbers on the chart of bond enthalpies but that should be the way to figure out which numbers to use from the chart. You can draw lewis structures to determine the number of bonds between the atoms in each compound.
Darth.Maul
Active member
Damn my touchscreen mobile device for being too slow.
*DUMBCAN*
Active member
Full solution. This took me way too long considering I'm a chemistry major... The last time I did one of these was four years ago, I'll use that as my admittedly dreadful excuse.
Three things have been cut off in the top-left - a hydrogen atom, and the other two read 1 x... Also that giant bracket in the bottom left shouldn't be that big...

Three things have been cut off in the top-left - a hydrogen atom, and the other two read 1 x... Also that giant bracket in the bottom left shouldn't be that big...

Bombogenesis
Active member
So glad I'm done with school and specifically the fucking chem sequence
Wompuscat
Member
Meh, all this crap in chem sucks. Hated Principles 1 and 2. Organic Chem is so much more fun. And way easier if you ask me. Actually learn the reactions though, most people just memorize shit. If you actually understand how the molecules flow and move, it makes the class so easy.
*DUMBCAN*
Active member
Well... I think you should just push through and learn principles. It's boring of course, but it helps so much. Though that all depends on what you're planning on doing with chemistry, if you're not taking it further it probably doesn't matter. If you hate principles then you'd hate chem at university, it's 90% principles.
Wompuscat
Member
I'm a senior at the University of Iowa. I've taken Principles 1 and 2 with lab, Organic Chem 1 and 2, and a seperate Organic chemistry Lab. I'm also currently in biochemistry. I was going to take advanced organic chemistry this semester but opted for human pharmacology. I finished Principles 1 and 2 my freshman year of college, and have never used it since. I have to review it for the MCAT, but as a person that almost got a chemistry minor, I can say push through Principles of chem, but it is nowhere near 90% of what you learn at the university.
*DUMBCAN*
Active member
Depends how you apply what I said. I meant it like: 'good understanding of principles will make 90% of the course significantly easier to understand.'.
Examples: group theory, stereochem, pretty much all main group chemistry (a lot of inorganic), crystal structures (a lot more of inorganic), etc... I could go on for a while. The main question is where in chemistry do principles not really help as much? Theoretical chemistry, quantum mechanics, statistical thermodynamics stand out for me. These (ST excluded) are the fundamentals of principles, so principles are really the answer to TC and QM.
Examples: group theory, stereochem, pretty much all main group chemistry (a lot of inorganic), crystal structures (a lot more of inorganic), etc... I could go on for a while. The main question is where in chemistry do principles not really help as much? Theoretical chemistry, quantum mechanics, statistical thermodynamics stand out for me. These (ST excluded) are the fundamentals of principles, so principles are really the answer to TC and QM.
a_pla5tic_bag
Active member
probably cause there's no/little math. If any of you really want to to do serious chemistry, get very very good at math.